
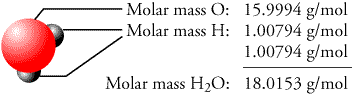
In the past, patrons of a cinema complex have spent an average.Using Table 8 below, determine the molar mass of sodium carbonate, Na2CO3.
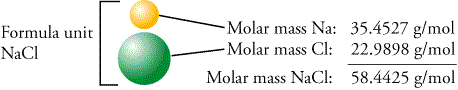
Table 7: Molar Mass of Calcium Chloride (CaCl2) Atomic Mass Multiply # in Contribution Formula Element Calcium (Ca) g/mol g/mol Chlorine (CI) g/mol g/mol Total = g/mol 3. Using Table 7 below, determine the molar mass of calcium chloride, CaCl2. Balance the following chemical reaction: Na2CO3 (aq) +CaCl2 (aq) → CaCO3 (s)+NaCl (aq) Na₂CO₃ + Call, CaCO3 + 2NaCl 2. Balance the following chemical reaction: Na2CO3 (aq) +CaCl2 (aq) → CaCO3 (s)+NaCl (aq) Na₂CO₃ +.ġ. What volume of seawater contains 5.0 kg of Seawater contains approximately 3.5%NaCl by mass and has aĭensity of 1.02 g/mL. Seawater contains approximately 3.5%NaCl by mass and has a.Many grams of NaCl (1 molar mass = 58.44 g/mol) are required to The given amount of NaCl (molar mass 58.44g/mol )and final calculate the molarity of each aqueous solution with the given amount of NaCl (molar mass 58.44g/mol )and final volumeĬalculate the molarity of each aqueous solution with.Hydrogen chloride is prepared using the reaction: NaCl(aq) + H2SO4(aq) → _HCl(9) + _Na2SO4(aq) What is the limiting reagent if 4.00 g H2S04 (98.09) is reacted with 393 moles NaCl (58.44) You must balance the equation first. How many oxygens are in the molecular formula? Include the number only. An acid with molar mass 116.08 g/mol contains by mass, 41.4 %0, 3.47 %H, and 55.1.Īn acid with molar mass 116.08 g/mol contains by mass, 41.4 %0, 3.47 %H, and 55.1 % Determine the molecular formula (must find empirical formula first).Is the molar mass of the solute in the second solution?
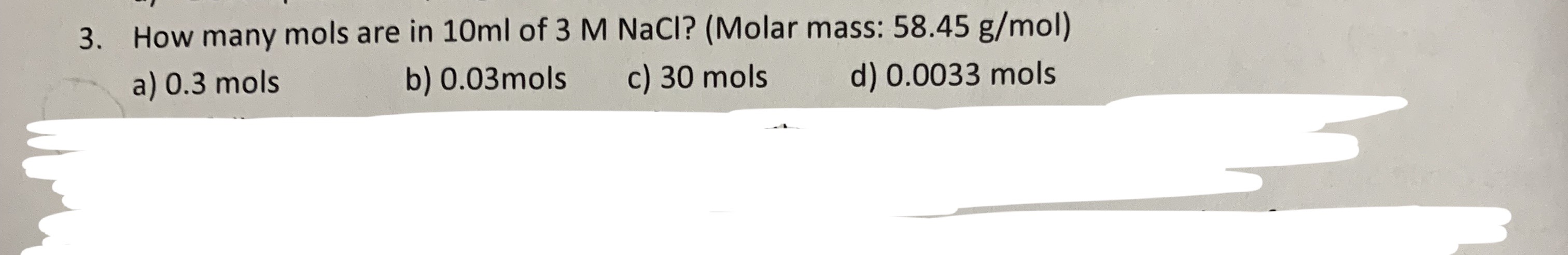
G/mol) is isotonic with 5.0% solution of non-volatile solute.


 0 kommentar(er)
0 kommentar(er)
